Welcome to 21st Century Lithium Production
LiTAS®
Lithium Ion Transport and Separation (LiTAS®) is EnergyX’s suite of lithium selective and processing mechanisms that incorporates proprietary mixed matrix membranes to improve lithium recovery for existing and new Li-brine resource producers. LiTAS® aims to process lithium from “Brine to Battery”.
Lithium’s unique characteristics provide enhanced performance benefits that are transforming the energy industry. Being a specialty material, cost-effective and time-efficient extraction processes remains the ‘holy grail’ for the EV transition.
Overview
LiTAS® is EnergyX’s portfolio of proprietary combination technologies including lithium selective, electrodialysis membranes. We provide customers the most economical lithium extraction process for their resource, and create sustainable solutions for battery grade lithium material products. EnergyX has designed and patented scalable implementation methods to cut costs and produce the lowest cost lithium in the market.

LiTAS® BREAKDOWN & IMPLEMENTATION
LiTAS® TECHNOLOGY COMPARISON
While current lithium technology on the market falls short in one area or another, LiTAS® is far more effective than any other technology currently available. Methods such as reverse osmosis can separate all ions out of water but are not selective. Nanofiltration can selectively separate ions, but cannot operate in high salinity environments (over 10% salinity) without dilution with freshwater. Other processes such as ion sorption and ion exchange can’t operate at high salinity, are a batch process versus a preferred continuous process, and require high amounts of fresh water and reagents with high power consumption.
| EnergyX Membranes | Ion Sorption | Ion Exchange Resin | Nanofiltration | Reverse Osmosis | Forward Osmosis |
|
|---|---|---|---|---|---|---|
| Selectivity Li vs Na | ||||||
| Selectivity Li vs Mg2+ | ||||||
| Operates at High Salinity | ||||||
| Continuous Process | ||||||
| Adaptable Platform | ||||||
| Enviromentally Neutral | ||||||
| Low Power Consumption | ||||||
| Non- Regenerative | ||||||
| No Freshwater Required |
Lithium refinery
EnergyX has developed breakthrough, nanotechnology membranes capable of nearly instant separation with high recovery rates. Our patented LiTAS® technology isolates the lithium from other impurities, and create a rich lithium chloride solution.
Initially we have built LiTAS® to complement the existing pond infrastructure in a phased approach. Eventually our direct lithium extraction technology will replace the outdated methods of evaporation ponds.
Conventional Ponds
LiTAS® Direct extraction

Energy Storage
EnergyX is positioning itself to be a major player throughout the energy storage value chain. As renewable energy demand soars, the need for low cost, large-scale energy storage systems is also rising.
Lithium batteries have been identified as a major part of the future of the energy transition. Their implementation in electric mobility and projects of various scales has shown off just how versatile they can be.
Overview
There are two elements in the global transition to renewable energy: energy generation and energy storage. First, the generation of renewable electricity from solar and wind has seen prices drop dramatically making them economically favorable. However, the generation of energy from these sources is extremely cyclical with reversion to fossil fuels during the down periods when the sun isn’t shining, or the wind isn’t blowing. Thus, energy storage from these resources is of the utmost importance to wane society’s dependence on fossil fuels. This is where the lithium batteries for portable and large-scale energy storage comes into play.

Battery Evolution
Just like the integrated circuit for computer chips, batteries continue to see step changes in their technological development. Lead-acid batteries were sufficient while the automotive industry thrived on the internal combustion engine (ICE). Lithium-ion batteries have enabled the transition to hybrid electric vehicles and all electric vehicles to begin, but they are not the long-term solution. Lithium metal batteries with their drastic improvement in energy density, safety, and charging times are the key to the future.
EV Revolution
The market for zero-emission, hybrid, and fully electric vehicles is estimated to be worth $912 Billion by 2026. IEA predicts there could be 130 million EV’s on the road by 2030. However, there are significant issues to be addressed related to the battery technology behind this push towards vehicle electrification. Batteries need to be further reduced in cost while improving their technical metrics related to energy density, charging time and cycle life.
Lithium Metal Battery Systems
EnergyX is lithium metal battery company working towards solving challenges related to current lithium battery technology. Lithium metal is the Holy Grail of anode materials for lithium batteries as it is the most efficient method of storing lithium within the cell.
During our preliminary research we have seen that lithium ions transport through our solid membrane materials at unprecedented rates. These results translate to extraordinary levels of conductivity and possible application as a solid state separator to enable lithium metal batteries.
Overview
Lithium metal is a transformational extension and optimization of lithium-ion batteries. Removing the graphite host material in the anode of the battery allows for batteries to be more energy dense by volume and by weight. Additionally, the direct platting of lithium at the anode removes the diffusion barrier of Li+-ions having to intercalate into the graphite host. All of these characteristics are paramount to improved function.
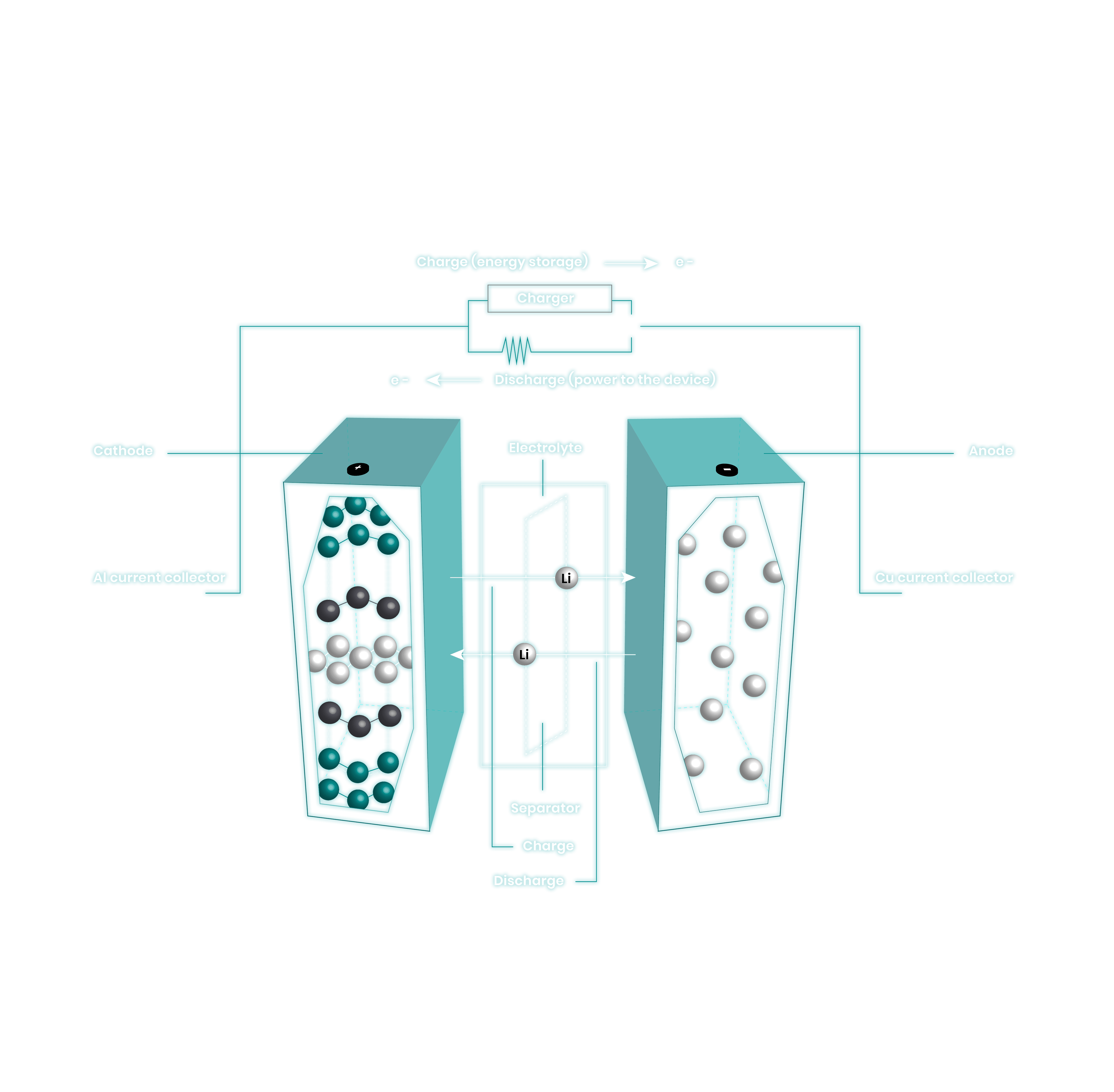
LITHIUM METAL VERSUS SILICON OR GRAPHITE ANODES
Lithium Metal is the most efficient way to store lithium within a battery. Although battery capacity is cathode limited, starting with a thin layer of lithium as the anode transitions the battery from a lithium deficient system – such is current Li+-ion batteries – to a lithium excess system allowing. This allows for longer cycle life as well as for any lithium ions consumed by parasitic reactions in the battery to be replenished by the small amount of excess lithium pre-deposited on the anode.
